
 Isabel Villegas, Senior consultant with Myrtle Consulting Group
Isabel Villegas, Senior consultant with Myrtle Consulting GroupIf you’re like most manufacturing companies, you’re operating in a business environment characterized by fluctuating raw materials costs and slimming margins. These factors have you continuously looking for ways to maximize time and resources, while also minimizing costs.
Efficient and effective product changeover, the process of converting the line or equipment in preparation for a switch from production of one product to another,
is one way that you can maximize the bottom line. Because of hesitation to risk an incomplete or incorrect line clearance, many manufacturers unnecessarily waste time and product quality. However, by building precision into the changeover process with the following six-step process, you can reduce waste and save money, all while ensuring product quality and safety.
Analyze Current Data
Perhaps the most critical element in ensuring the optimal product changeover process is data. You can’t manage what you can’t measure. Manufacturers often find that they are deficient when it comes to tracking narrow tolerances in the line clearance process, whereby a certain volume of usable product is run through the line to “wash out” traces of previous product. By using a simple control chart, you can note variability in product loss. Figure A provides a sample control chart, which reveals variance in product used for line clearance over time.
An expert control system will continually use a variety of charts to assess variation in process and product characteristics after collecting data. Chart rules are then referenced for making process adjustments. This removes dangerous and costly subjective tinkering with changeovers.
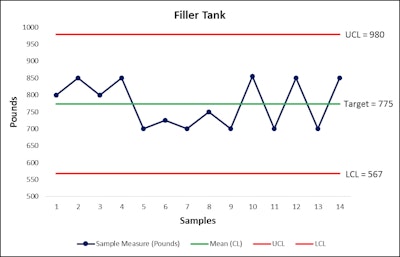 This control chart provides an example of how manufacturers can collect data on variation in the line clearance process.
This control chart provides an example of how manufacturers can collect data on variation in the line clearance process.Understand Legalities And Company Policies
While product changeover is important in any manufacturing process, it is highly critical in some cases, particularly where food allergies are concerned. According to a study released in 2013 by the Centers for Disease Control and Prevention, food allergies among children increased approximately 50% between 1997 and 2011.[i] The Food & Beverage (F&B) industry in particular, is faced with increased responsibility to ensure a safe product for consumers with allergies. Not only are product changeovers an important activity for ensuring product quality, the Food and Drug Administration (FDA) requires them.
It’s important to understand and document company policy, labeling guidelines and FDA regulations surrounding changeover requirements, especially as it relates to allergens. With these in mind, you can target adequate minimal levels necessary for line clearance and/or modify labels to ensure that products are safe.
Determine Targets
One data point is not sufficient to design a precise line clearance target/process. The process of diverting and measuring discarded product must be conducted on different manufacturing lines and/or for different products. In many cases, the volume of product discarded is not the same for every manufacturing line. In one notable case, the volume of product wasted ranged from 400 – 1,900 pounds, a red flag indicating that there was significant room for improvement in the flushing process.
Extensive data is required for setting the optimal line clearance targets. Effective root cause analysis, statistical process control and process capability methods can be used to determine optimal targets for line clearance rates for a given process. Even though the shifted target is based on actual data, it does require monitoring to ensure the desired results are achieved.
Post Control (Tracking) Charts
Throughout the process, it’s important to post control charts on the plant floor and record data on a regular basis. After the target results are validated by Quality Assurance and the recommended target is implemented as a permanent change to the process, communication and tracking on the production floor by means of a tracking chart are necessary. Continued use of control charts helps to monitor performance with regard to increasing line clearance consistency. Analysis may also reveal other potential efficiencies such as time savings that can be generated by scheduling runs in a way that minimizes sanitation and packaging changes between batches.
Install Meetings
Data without action is not sufficient to change behaviors. It’s important to establish meetings to evaluate reported data and identify actionable items as a team. By establishing regular meetings by shift, weekly and on a monthly basis (as required), it’s possible to review control and tracking charts, understand variances and set goals. Reviews on a shift basis are used to determine common and special causes of any outliers in the process. Based on this information, your team can determine what actions need to take place to minimize variation in the process. All organizations’ management operating systems contain feedback and control loops to promote continuous improvement of processes as illustrated by the prior tracking chart. The current shift tracking results are aggregated and should be discussed in the pre-shift meetings so that the team can highlight possible causes for data points that fall outside the standard deviation. If each shift is managed properly with respect to line clearance levels, then the sum of the shifts will result in favorable weekly, monthly and yearly performance. The aim of these meetings is also to standardize the execution process. Monthly meetings should be held to track results, ensure sustainable progress and detect further opportunities occurring over time.
Review Results And Current Targets
Measuring and monitoring must continue on a regular basis to maintain achievements with regards to optimal flushout processes.
Failure Modes and Effects Analysis (FMEA) is a systematic, proactive method for evaluating a process to identify where and how it might fail – and to assess the relative impact of different failures in order to identify parts of the process that most need adjustment. While “analysis” is a key part of this methodology, FMEA can also be used to develop implementation standards for adjusting and repairing a subpar changeover and line clearance process.
Using FMEA, you can evaluate processes for possible failures and determine ways to prevent adverse events by proactively correcting or automating a process rather than reacting to failures after they occur (firefighting). This emphasis on prevention may reduce risk of product mixing or contamination while also limiting product loss. FMEA is particularly useful in evaluating a new process before implementing it to assess its potential impact on an existing process.
Conclusion
Before you can reduce waste associated with changeover, you first must use data to understand the problem. Embracing a method for perfecting the changeover and line clearance processes is not easy. Be prepared to lose some product in the testing process and to invest some time in setting up the evaluation process. Keep in mind that product discarded during this optimization process will generate a return in the form of salvaged commercial grade product from every single batch after the study is completed.
Not only does an optimized product changeover process help industries like F&B and Consumer Packaged Goods (CPG) comply with FDA regulations, it also translates into putting good product to use instead of simply wasting it.
Isabel Villegas is a senior consultant with Myrtle Consulting Group. She has extensive experience in process improvement and change management within local government and private corporate entities, including areas of the value chain in both private and public sectors.























