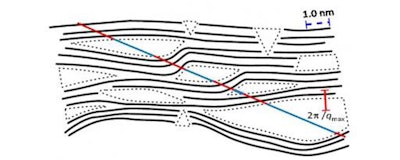
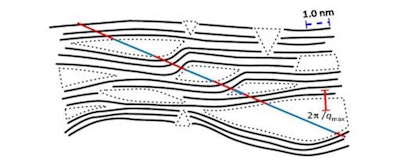
 Schematic view of carbon structures with pores. (HZB)
Schematic view of carbon structures with pores. (HZB)Optimized nanoporous carbons can serve as electrodes for fast electron and ion transport or improve the performance of energy storage and conversion devices. Thus the tunability of the size, shape, and distribution of pores is highly required. The team at the HZB Institute for Soft Matter and Functional Materials collaborated with a group at the University of Tartu, Estonia, to inquire the nanoarchitecture, inner surface, size, form and distribution of nanopores in dependence of the synthesis conditions.
Colleagues in Estonia produced a series of nanoporous carbons by reacting a powder of molybdenum carbide (Mo2C) with gaseous chlorine at 600, 700, 800, 900, and 1000 degrees Celsius. Depending on the synthesis conditions chosen, the nanoporous carbon exhibit different properties such as surface area, porosity, electronic and ionic conductivity, hydrophilicity, and electrocatalytic activity.
Surface structures were analysed by transmission electron microscopy at the HZB. The interior surface area of nanocarbon materials is usually investigated by adsorption of gas. However, this method is not only comparatively inaccurate, it also contains no information about the shape and size of the pores. For deeper insights, Dr. Eneli Härk and her colleagues at HZB worked with small-angle X-ray scattering, a technique permitting to obtain information on various structural features on the nanometer scale including the mean pore size.
Small-angle X-ray scattering not only provides information on the precise inner surface area and the average pore size, but also on their angularity, i.e., sharp edges of formed pores, which play a major role for the functionalization of the materials. "The SAXS analysis summarizes over an enormous amount of micropores omitting misleading assumptions thereby directly relating the nanostructural architecture of the material to macroscopic technical parameters under investigation in engineering" Härk explains.
The main aim was to understand structural formation, and electrochemical characteristics of carbon as a function of the synthesis temperature. "For optimal function, not only the high inner surface area is crucial, but the pores should have exactly the right shape, size, and distribution," says Härk.
(Source: Helmholtz-Zentrum Berlin Für Materialien Und Energie)






















